Separate and combine substances
...explore: Create your own jewellery
The galvanization goes back to the doctor "Luigi Galvani" who discovered this phenomenon the 6th of November 1780.By galvanization we mean the whole process where the metals are separated electrochemically in a conducting liquid (electrolyte) and transferred to other metal objects. The electricity conducting liquid is an aqueous solution of the metal destined to cover the object. In our experience, we dissolve copper sulphate in water to copper a metal plate.
For that, the positive and negative poles are plunged in a copper sulphate solution and connected to a constant tension source (e.g. battery). Under the influence of the electric current, the small positive copper particles (copper ion) are released and move to the metal plate on the negative pole. They then cling to the metal plate and cover it with a thin layer of copper.
Note : Dispose of the copper sulphate solution in a particular chemical disposal unit e.g provided by the municipality.

1

A mysterious coating
Thanks to chemical products, coat your metal plate with a mysterious gold coating.

2
Material
Container with copper sulphate and citric acid
3 1.5V batteries
Ring magnets, paper clip
Metal plate
Connectors with pliers
Hard foam stick
Glasses kit, latex rubber gloves
Cardboard box part, foil, white paper
Adhesive tape, scissors, plastic bag

3
Cut out the glasses parts and assemble them with some tape.

4
Modify the length of the temple along the dots so the glasses hols well.

5
Create with the top of the box, a laboratory basin. Take the plastic bag to protect the rest of the material.
Lay a plastic film inside the cardboard box and flatten it out.

6
Cover the bottom of the box with a white sheet.
Lay the glasses and gloves within easy reach.

7
Prepare the solution
Put the glasses and gloves on.
Open the copper sulphate and citric acid container in the basin.

8
Fill three quarters of the container with water (yellow arrow).
Screw the lid on and shake the container to dissolve the powder.
9
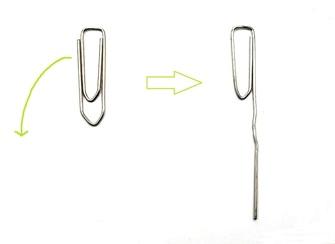
Unfold the wire downwards (green arrow)

10
Place the clip like shown on the picture. The bended part dips in the water!
The sulphate solution has now a blurry and bluish aspect.
11
Assemble the 4.5 Volt tension source
- For the copper galvanization, we need a 4.5 Volt tension source. But we only have 1.5 Volt batteries. How can we do it? Who finds a solution?
- Cut a 14 cm piece out of the foam stick.
12
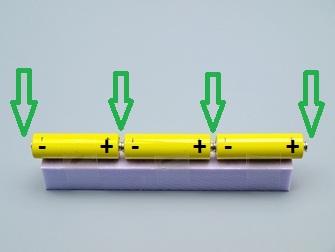
Link the batteries with two ring magnets. Put two other magnets on the free poles (green arrow).
Careful: The positive pole (+) must always be in contact with the negative pole (-) of the beside battery. (series circuit)
Fix the batteries to the foam with tape.
13
Prepare the galvanization
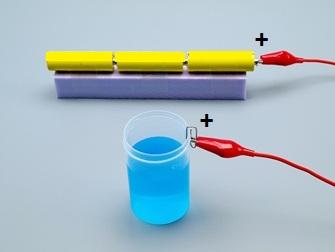
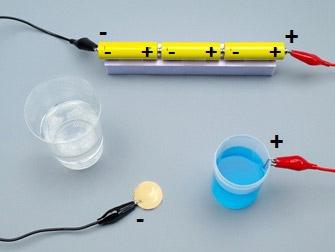
Connect the positive pole (+) of the tension source to the clip in the container.

14
Make sure the plier holds on the clip well!

15
16

Galvanisation
Connect the metal plate to the negative pole (-) of the tension source.

17
Completely dip the metal plate in the blue copper solution.
As soon as the plate is in the solution, bubbles appear around the clip...

18
Careful!
The plate mustn't touch the clip.
The plate must constantly be in movement and the solution stirred.

19
Check now and then the colour change of the plate.

20
Take the plate out after one minute, rinse it with water and dry it.
| Views | |
|---|---|
| 29 | Total Views |
| 29 | Members Views |
| 0 | Public Views |
Share by mail
Please login to share this webpage by email.